Eukaryotic cells control the assembly of cytoskeleton filaments into structures ranging from networks, where filaments cross at large angles, to bundles, where filaments are closely packed and nearly parallel to one another. These higher-order structures are of fundamental importance to cell functions. For instance, while actin bundles are crucial in cell locomotion and adhesion, actin networks control the cell surface’s mechanical properties. Cells can switch between the formation of these two structures during cell crawling and division. Linker proteins and multivalent cationic ions such as Ca and Mg control cytoskeleton filaments’ morphology, structure, and stability. Certainly, cross-linking proteins are not always required for bundle formation when sufficient filament surface charge neutralization can directly induce bundling due to charge inversion. When cytoskeleton filaments are immersed in an aqueous medium, they reveal a surface charge regulated by protonation/deprotonation reactions of the dissociable functional groups at the solid/liquid interface. The accumulated charge depends on their tertiary molecular structure and polymerization state. For instance, tubulin and G-actin isoforms have different charges according to their amino acid sequence. Thus, filaments formed by these isoforms might be more susceptible to bundling at borderline conditions. In a similar vein, mutations reduce the charge of actin and have reportedly caused the mutant filaments to bundle spontaneously.
Additionally, the strong electrostatic interactions between the filament and aqueous medium lead to an electric double layer formation. In this tiny, strongly correlated liquid volume, three competing / balanced molecular interactions (water crowding, electrostatic screening ion correlation, mean electric potential) play a crucial role in the bundle formation at low filament concentrations. For instance, the surface electric potential’s magnitude brings detailed insight into the dispersion mechanism and filament stability. When the biological fluid completely neutralizes the filament charge, the surface electric potential (or the surface charge) of the filament becomes zero, and cytoskeleton filaments can easily form bundles due to the absence of inter-filament repulsive forces. However, how the pH level, electrolyte concentration, ionic species type, and molecular structure conformation affect the cytoskeleton filaments’ surface electric potential is still poorly understood. It was recently introduced an innovative multi-scale approach (JACFC) that can account for the atomistic details of the protein molecular structure, the biological environment, and its impact on the surface electric potential of cytoskeleton filaments. It is a powerful tool to characterize cytoskeleton filaments’ bundling formation properties under different ionic environments such as ionic strength, pH, ion type, and molecular structure conformations.
User Guide
usserguideStability
Snapshot Gallery
 |  |
 |  |
Theory
CSDFT_theory_from_SC1_proposal
More sophisticated theories are required to characterize bundles and network structures of cytoskeleton filaments controlled by linker proteins and at high filament concentrations. In this case, inter-filament interactions and linker proteins properties play a crucial role in cytoskeleton filaments’ structure and stability. For instance, fimbrin is capable of cross-linking actin filaments into bundle assemblies independent of concentration. On the other hand, fascin, a fairly abundant protein, is concentration-dependent but maintains the ability to cross-link bundles efficiently and with substantial resistance to shear forces. This approach will be included in future updates on JACFC.
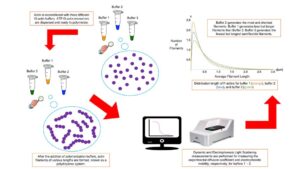
High-Quality Actin Filament Sample Preparation for Light Scattering Measurements
Available in vitro experimental data on actin filaments often lack details and information on sample preparation protocols and experimental techniques, leading to unreproducible results. Additionally, different experimental techniques and polymerization buffers provide different, sometimes contradictory results on the properties of these systems, making it substantially difficult to gather meaningful data and conclusive information from them.
Modern dynamic light scattering (DLS) and electrophoretic light scattering (ELS) instruments are the most accurate, non-invasive, experimental techniques to characterize hydrodynamic and electrostatic properties of polydisperse charged biomolecules even at low concentrations and using small sample volumes. These instruments use advanced technology and multi-functional software to measure the hydrodynamic size, polydispersity, dispersion stability and aggregation, biomolecular charge, and zeta potential of particles and polymers immersed in aqueous biological environments. To ensure high accuracy and reproducibility of the results, it is essential to use high-quality samples, accurate instruments, robust software, and optimized measurement protocols.
We have recently introduced a robust, accurate, detailed polymerization protocol to prepare high-quality actin filament samples for light scattering experiments. It has provided unicity and consistency in preparing stable, dispersed, aggregates-free, homogenous actin filament samples.
Illustration
Bio-Protocol
Bio-protocol4553
Impact of Actin filament polydispersity and semi-flexibility on their hydrodynamic, mechanical, and electrostatic properties
Due to their hydrodynamic, mechanical, and electrostatic properties, cytoskeleton filaments have the extraordinary ability to dynamically change conformations in response to alterations in G-actin concentration and type of crosslinker/binding proteins and electrolyte concentration. These cytoskeleton properties are crucial for eukaryotic cells to achieve specific biological functions in different cellular compartments, which may vary depending on the cell type and location conditions. While a substantial amount of research has been done in the biochemistry and biophysics fields, the underlying principles and molecular mechanisms that support these filaments’ hydrodynamic, electrostatic, and stability properties remain elusive.
Most conventional methods for biopolymer solutions center on rigid, monodisperse, and sometimes uncharged cylindrical models and theories. These approaches may be inappropriate for cytoskeleton filaments because they omit essential hydrodynamic and polyelectrolyte filament properties. We extended those approaches to account for filament polydispersity and semi-flexibility impact on actin filaments’ translational diffusion coefficient and electrophoretic mobility properties. An asymmetric, exponential length distribution for hydrodynamic conditions characterizes the actin filament polydispersity and the disparate rate lengths of barbed and pointed ends. A modified cylindrical wormlike chain model was also used to describe the filament semi-flexibility, effective monomer charge, and diameter. We considered typical experimental values for the degree of polymerization (370 G-actin proteins per um) and associate rates (barbed end ten times larger than the pointed end). The values for the other parameters were adjusted to reproduce the experimental data. This characterization is innovative since these parameter values are obtained from non-invasive experiments using the same experimental and hydrodynamic conditions.
We considered three different buffers, g-actin, and polymerization, used in previous works to elucidate the impact of their chemical composition, reducing agents, pH values, and ionic strengths on the filament properties. Compared to those values obtained from molecular structure models, our results revealed a lower value of the effective G-actin charge and a more significant value of the effective filament diameter due to the formation of the double layer of the electrolyte surrounding the filaments. Additionally, compared to the values usually reported from electron micrographs, the lower values of our results for the persistence length and average contour filament length agree with the significant difference in the association rates at the filament ends that shift sub-micro lengths—the maximum of the length distribution. More details can be found below.
polymers-14-02438-v2(2)
Density Functional Theory for Polydisperse, Semiflexible Cytoskeleton Filaments
An increase in the density of G-actin/tubulin and electrolyte concentration can lead to conformation transformations from the orientation-disordered (isotropic) to orientation-ordered (nematic) phase and increase the filaments’ average length. Additionally, a growth in the number density of binding agents, such as divalent ions or linker proteins, can yield bundling or network conformations. These self-organization behaviors, yet poorly understood, have been observed experimentally. Currently, valuable information on the distribution and type of cytoskeleton conformations in cells is obtained from fluorescence and electron microscopy images, whereas confocal microscopy captures their dynamic conformation changes. However, this information usually provides an incomplete molecular understanding of the interplay between the polydispersity, semi-flexibility, polyelectrolyte, and mechanical properties of cytoskeleton filaments on their conformational dynamics, self-organization, and stability.
Much theoretical research has been performed to study the isotropic to nematic phase transformation in macromolecule solution. The conventional understanding of the properties of these polyelectrolytes is based on monodisperse (e.g., same filament lengths), mean-field theories, and rod-like cylindrical filament models (e.g., with contour lengths shorter than their persistence length). These methods break down for cytoskeleton filaments because they entail several approximations to treat the inter-filament interactions, electrolytes, and filament structures.
As a first step to face this challenge, we introduced a novel density functional theory for polydisperse, semiflexible cytoskeleton filaments. The approach accounts for the equilibrium polymerization kinetics, length and orientation filament distributions, and the electrostatic interaction between filaments and electrolytes. As a unique feature, the formulation can determine critical parameter values governing the isotropic–nematic phase diagram behavior. This approach is essential to study the self-organization behavior of actin filaments in different cell compartments, where the G-actin polymerization and electrolyte conditions may generate filaments with different conformations and lengths long enough to become semiflexible. We considered experimental conditions on the actin filaments’ solution where the isotropic–nematic phase diagram transition is due to changes in the G-actin concentration. The filament average size for different G-actin concentrations was also fixed by changing the gelsolin proteins’ concentration. We obtained the size distribution function from these special equilibrium conditions, whereas we used Sluckin’s trial function to calculate the angular distribution function in the nematic phase. Additionally, we introduced an ansatz for the standard chemical potential excess of actin filaments that results in the asymmetric Schulz distribution function for the actin filaments size. This distribution function agrees with those used in light-scattering experiments on actin filaments. In addition, we generalized the formula for the orientational free energy to the case of a polydisperse system to account for the filament’s semi-flexibility. Finally, we calculated the isotropic–nematic phase diagrams for various Schulz size distributions, persistence lengths, and concentrations of monovalent ions in the electrolyte solution. More details can be found below.
polymers-14-02042-v2(1)