Experimental research under in-vitro conditions revealed that cytoskeleton filaments could conduct ions from one filament end to the other when submerged in an aqueous electrolyte solution. These ionic currents are related to the condensation of counterions in the diffusive layer of the electrical double layer, which moves freely along these filaments. They have been investigated theoretically using non-linear electric circuit transmission line models, revealing slow localized traveling waves (solitons) along cytoskeleton filaments. This finding may provide new insights into many electrical processes taking place within smooth muscle, skeletal, and cardiac cells near the membrane or in the nucleus. Furthermore, an additional information delivery system in neuronal cells might benefit intracellular communication in dendrites, the soma, axon, and axon terminal. Indeed, further clarification on the electrical conductivity and ionic transmission properties of actin filaments is vital for a complete understanding of their contributions to cellular functions and information transfer inside cells.
JACFC: A multi-scale approach to characterize Monovalent Diffuse Ions Along Actin Filaments in physiological conditions
A multi-scale approach (atomic(Å) → monomer(nm) →filament(μm)) was introduced to account for the atomistic details of a protein molecular structure, its biological environment, and its impact on electrical impulses propagating along wild-type actin filaments. The formulation includes non-trivial contributions to the ionic electrical conductivity and capacitance coming from the diffuse part of the electrical double layer of G-actins.
Multi-Scale Model
- Atomic(Å) → Monomer(nm). A filament molecular structure was used to characterize the cylindrical biomolecular model’s effective radius and surface charge for G-actin proteins. This model for monomers and an extension of the Poisson-Boltzmann theory called CSDFT for aqueous electrolyte solutions were used to describe the radial electrical potential and charge accumulation layers around the surface (CSDFT software can be downloaded from this GitHub website). These results and a suitable modification of Nernst-Planck theory were used to account for the monomer (radius) size and surface charge density as well as solvent dielectric and viscosity, ion condensation, and pH level impact on the ionic inductance L, radial capacitance Co and axial Rl and radial Rt flow resistances characterizing the electrical circuit model for a single monomer.
- Monomer(nm) →Filament(μm). We use the monomeric electric circuit unit in a nonlinear inhomogeneous transmission line prototype model to account for the monomer–momomer interactions, dissipation, and damping perturbations along the filament length. A well-known differential equation for the voltage and current along the filament is obtained using different approximation levels.
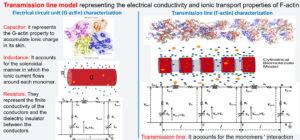
The approach revealed the propagation of electrical signal impulses in the form of solitons for the range of voltage stimulus and electrolyte solutions typically present for intracellular and in vitro conditions. The approach predicts a lower electrical conductivity with higher linear capacitance and non-linear charge accumulation for intracellular conditions. The results show a significant influence of the voltage input on the electrical impulse shape, attenuation, and propagation velocity. The filament can sustain the soliton propagation at almost constant speed for the in vitro condition. In contrast, in the intracellular condition, it displays a remarkable deceleration. Additionally, the solitons are narrower and travel faster at higher voltage input. More information can be found below.
ElectricalSignalPropagation
JACAF software
The desktop application can be downloaded from our GitHub repository website. The Java web application, also available from this website, provides experts and non-experts in the field with a visualized guide (graphical user interface) to perform these calculations online without computational cost.
Snapshot Gallery
 |  |
 |  |
User Guide
usserguidesolitons-1
EIAF: A multi-scale approach to characterize Monovalent Diffuse Ions Along Actin Filaments in pathological conditions
In this study, we have extended the previous approach to characterize the electrical impulses along actin filaments in both muscle and non-muscle cells for a wide range of physiological and pathological conditions. This computational Mathematica notebook tool offers an interactive component that allows investigators to choose the experimental conditions (intracellular Vs. in vitro), nucleotide state (ATP Vs. ADP), actin isoform (alpha, gamma, beta, and muscle or non-muscle cell), as well as a conformation model that covers a variety of mutants and wild-type (the control) actin filament. We analyzed environmental changes such as temperature effects, pH changes of the surrounding solutions, and structural changes to an actin monomer due to radius changes. Additionally, we investigated the electrostatic consequences of actin mutations from different disease conditions for the first time.
Temperature changes and pH differences, which are known to occur in unhealthy muscle and non-muscle cells, were shown to result in different ion accumulations at the surface of the actin monomer (and filament), ionic conductivities, and ionic soliton wave packet velocities. The changes in radius showed a competition between the ion velocity profiles and the resistance when influencing the soliton propagation velocity. When comparing the results of pH changes and actin mutations, common trends resulted in both analyses. Specifically, there were impacts on the ion concentration profiles, current density, and traveling ionic solitons. This is because pH fluctuations in the solvent change the charge of the protein. For instance, the charge of the Cong model unit is -83e, -154e, and -184e for pH values 6, 7, and 8, respectively. That represents approximately -6e, -11e, and – 13e charges per monomer for increasing pH. In the analysis of mutations, we used -8e, -9e, and -12e for the mutated actin monomeric isoforms ACTB1, ACTA1, and ACTA2. This leaves an open question regarding the connection between the pH value in the intracellular space of cells and diseases involving missense mutations with at least one of the seven pH-dependent amino acids. Despite a missense mutation only replacing one amino acid on an actin monomer, the ionic wave packet velocity results show a consequential outcome. These studies provide an unprecedented molecular understanding of why and how age, inheritance, and disease conditions may induce dysfunctions in the biophysical mechanisms underlying the propagation of electrical signals along actin filaments. Download the Mathematica script EIAF from our GitHub repository website. More information can be found below.
Illustrations
 |  |
Snapshot Gallery and User Guide
CPC2022_red_sizeElectrical Propagation of Condensed and Diffuse Ions Along Actin Filaments
Experimental evidence on multivalent ions has demonstrated that modeling the electrical double layer (EDL) around cytoskeleton filaments becomes inaccurate due in part to an overestimation of the relative dielectric permittivity of the aqueous medium, a lack of consideration of immobile water molecules near the surface, and the nonlinear behavior of the mean electrostatic potential used in EDL theories for distances near the protein surface. In this work, we elucidated the roles of divalent ion condensation and highly polarized immobile water molecules in propagating ionic calcium waves along actin filaments. We introduced a novel electrical triple-layer model and used a non-linear Debye-Hückel theory to characterize the physicochemical properties of G-actins.
Condensed and Diffuse Electrolyte Layer Model
 |  |  |
We considered the neuron resting and excited states using representative mono and divalent electrolyte mixtures. Additionally, we used 0.05V and 0.15V electrical voltage stimuli to study ionic waves along actin filaments in voltage-clamp experiments. The results revealed that the physicochemical properties characterizing the condensed and diffuse layers lead to different electrically conductive mediums depending on the ionic species and the neuron’s state. This region-specific propagation mechanism might provide a more realistic avenue of delivery through cytoskeleton filaments for larger charged cationic species. A new direct path for transporting divalent ions might be crucial for many electrical processes taking place in ion channels, the dendrites, axons, terminals, and the soma. For instance, actin filaments can alter calcium influx at membrane voltage-gated ion channels and regulate calcium release deep in the nerve at the endoplasmic reticulum. Additionally, actin is a pathway for calcium to the stereocilia of the inner ear and is involved in cellular calcium signaling by storage and release. Download the Mathematica script from this GitHub repository. More information can be found below.
JCN2022-red-size
CPPCAF: A molecular structure model for actin filaments
In this research project, we have extended the cylindrical biomolecular model for actin filaments to account for the surface roughness on their mean electrostatic potential (MEP) and the total ionic current density (TICD). We used COMSOL software to numerically calculate and visualize these properties under different electrolyte conditions and longitudinal voltage inputs.
In illustrations 1 and 2, we display preliminary data on MEP and TICD for 100 mM KCl and 100 mM KCl + 25 mM CaCl electrolytes at pH 7, respectively. Adding calcium dichloride to potassium chloride enhances the electrical conductivity, consequently increasing MEP and TICD on the filament’s surface.
 Figure 1 |  Figure 2 |
In illustrations 3 and 4, we also show preliminary data on MEP and TICD for 100 mM KCl and 100 mM KCl + 25 mM CaCl electrolytes at pH 6, respectively. Lowering pH to 6 diminishes the electrical conductivity, decreasing MEP and TICD on the filament’s surface.
 Figure 3 |  Figure 4 |
It was found that the irregular shape of the filament structure model generated pockets, or hot spots, where the current density reached higher or lower magnitudes than those in neighboring areas throughout the filament surface. It also revealed the formation of a well-defined asymmetric electrical double layer with a thickness more prominent than that commonly used for symmetric models. More information can be found below.
RSC_adv2022_reduced_size
CPPCAF software
CACPPAF is a COMSOL Multiphysics tool for educators and researchers. It is available from this website and provides experts and non-experts in the field with a visualized guide (graphical user interface) to perform these calculations online without computational cost. This tool allows expert and non-expert users to numerically evaluate the electric potential, ionic concentration distribution, velocity profile, and ionic current along a molecular structure surface characterizing actin filaments. As a unique feature, our software accounts for surface roughness on the filament surface, the finite filament size, and the ion condensation effects on these polyelectrolyte properties. CACPPAF uses a molecular structure model for wild-type actin filaments to increase accuracy and implements the solution for non-linear PB without substantially increasing the computational cost. Additionally, the user can change predefined input parameters such as the temperature, voltage input, ion concentrations, and filament surface charge density to study actin filaments in various conditions. The software does not require knowledge of specific programming languages or expertise in computational modeling and comes with a detailed user guide. Additionally, users can download the application to their local machines from our GitHub repository website. Running the application locally requires users to own COMSOL Multiphysics and Chemical Reaction Engineering Module licenses. Additionally, the performance will highly depend upon their machine’s capabilities. More information can be found below.
Illustrations
Snapshot Gallery and User Guide
softwarex2022